Details of the Drug
General Information of Drug (ID: DMWVIGP)
| Drug Name |
Digitoxin
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acedoxin; Asthenthilo; Cardidigin; Cardigin; Cardiolanata; Carditalin; Carditoxin; Coramedan; Cristapurat; Crystodigin; Digicor; Digilanid; Digilong; Digimed; Digimerck; Digipural; Digisidin; Digitoksim; Digitoksin; Digitophyllin; Digitossina; Digitoxina; Digitoxine; Digitoxinum; Digitoxoside; Digitoxosidum; Digitrin; Ditaven; Glucodigin; Lanatoxin; Lanostabil; Monodigitoxoside; Myodigin; Natigal; Pandigal; Panlanat; Purodigin; Purpurid; Tardigal; Unidigin; Crystalline digitalin; Digitaline cristallisee; Digitaline nativelle; Digitalinum verum; Digitossina [DCIT]; Digitoxigenin tridigitoxoside; LT00244784; Crystodigin (TN); De-Tone; Digitalin, crystalline; Digitaline (TN); Digitoxigenin-tridigitoxosid; Digitoxigenin-tridigitoxosid [German]; Digitoxina [INN-Spanish]; Digitoxine [INN-French]; Digitoxinum [INN-Latin]; Mono-digitoxid; Mono-digitoxid [German]; Mono-glycocard; Purodigin, crystalline; Tri-digitoxoside; Tri-digitoxoside [German]; Digitoxin [INN:BAN:JAN]; Digitoxin (JP15/USP/INN); Inhibits Na+/K+ ATPase; 5.beta.-Card-20(22)-enolide, 3.beta.,14-dihydroxy-, 3-[tris-(digitoxoside)]
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiarrhythmic Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
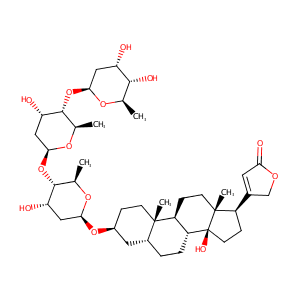 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 764.9 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Digitoxin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
